(HYPERTEXT SAMPLE)
Welcome to the World of Chemistry:
Where everything around us; surrounds with particles that are unseen to the naked eyes.
Since this is my first time to make a blog so I will make some adjustments from time to time.

To be honest with you, the way I talk is considered to be informal. I was going to share with you my powerpoint document though there were some technical issues had happened. So my best solution is just to take some screenshots of my document for you guys to see.
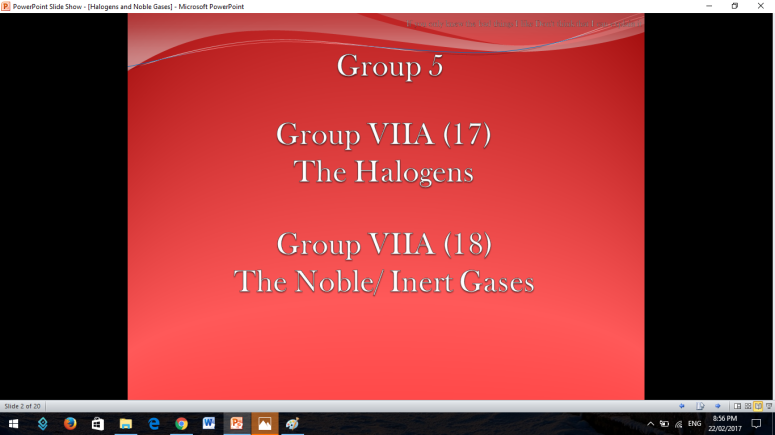
As you can see: 2 families are only ones to be given emphasis since this was just our last report in our General Chemistry I subject.
Group 17 and 18 are located on the right side of the periodic table of elements so there are both non-metals.
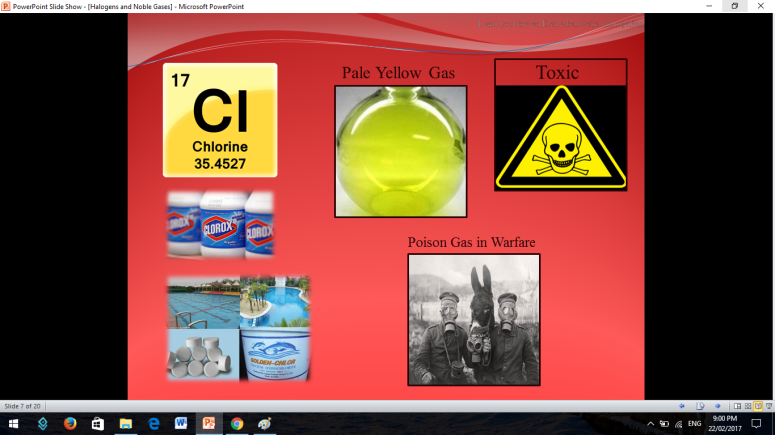
The first thing that is going to be discussed are the Halogens
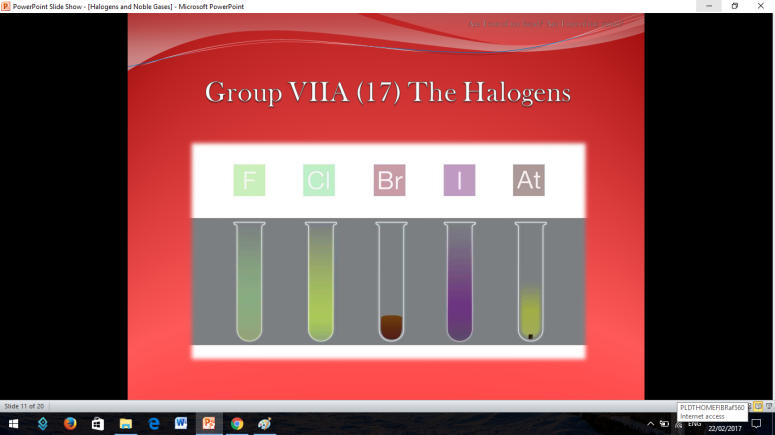
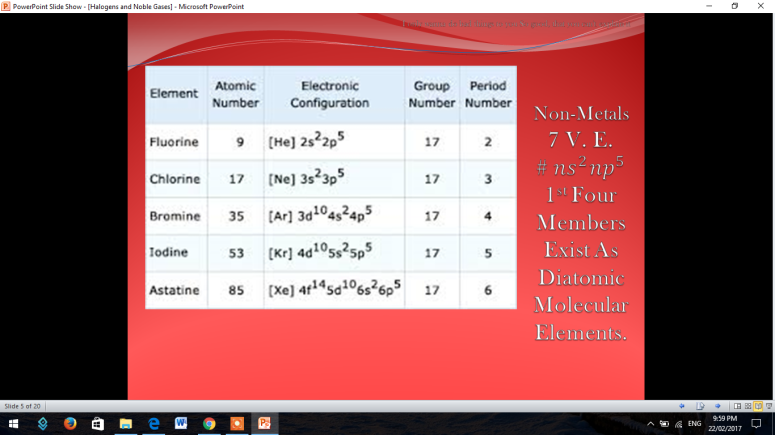
To fully understand the the halogens, there is a short video for you to be witnessed.


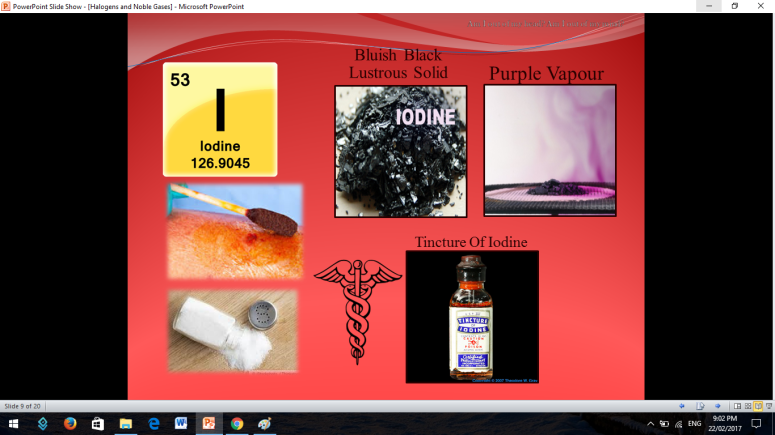
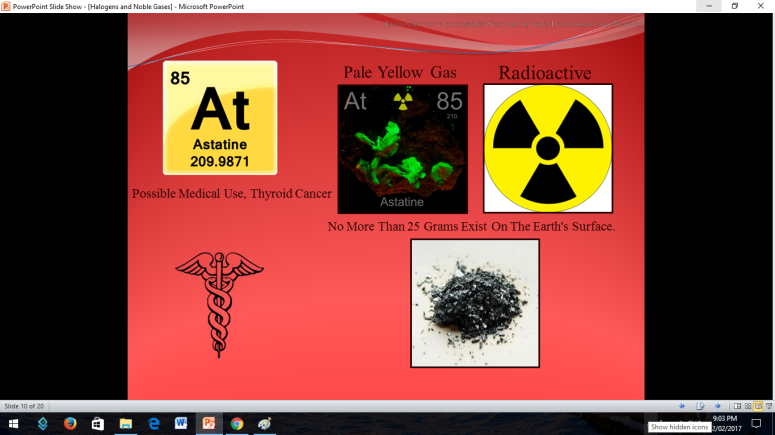
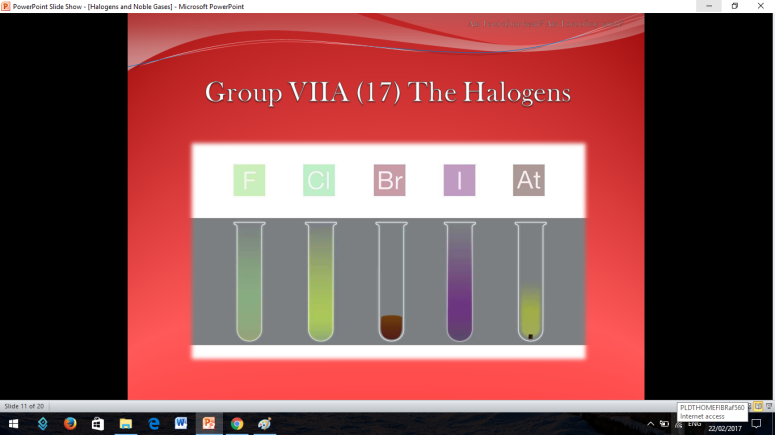
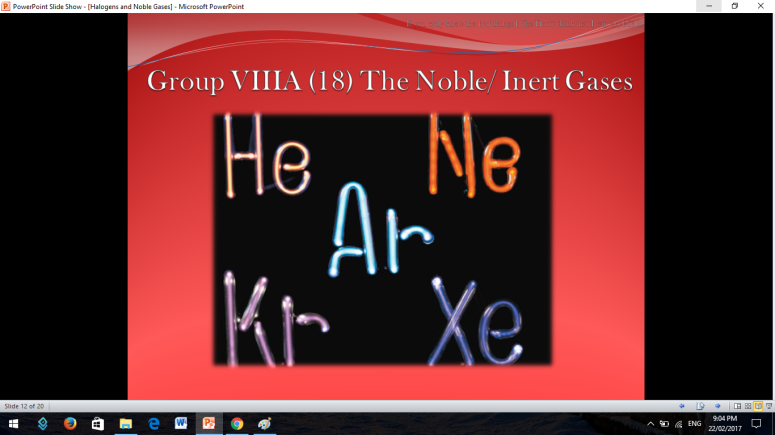
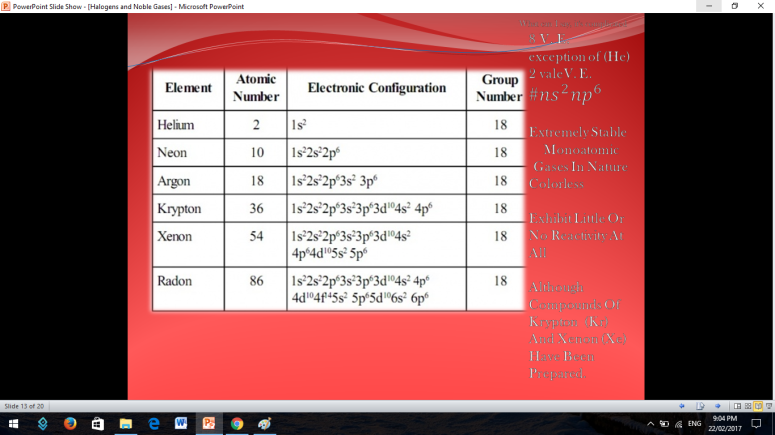
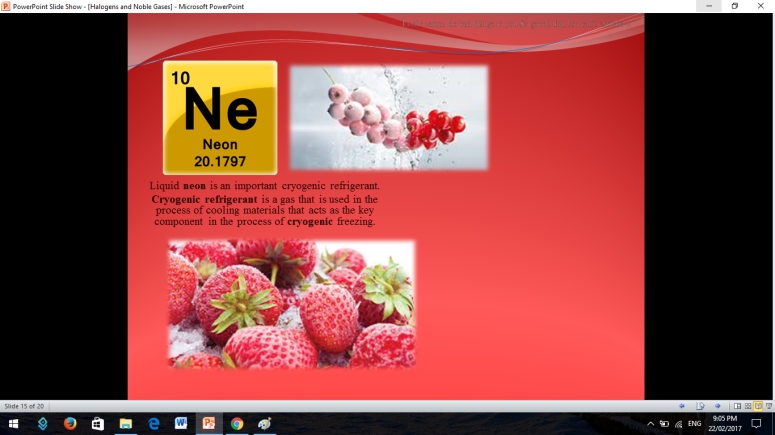
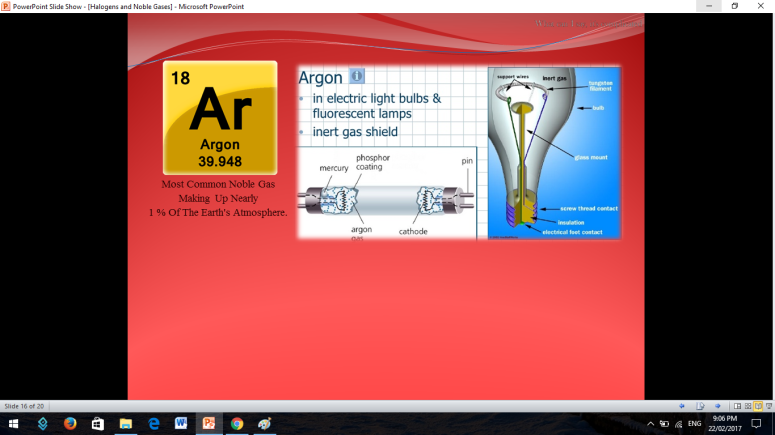
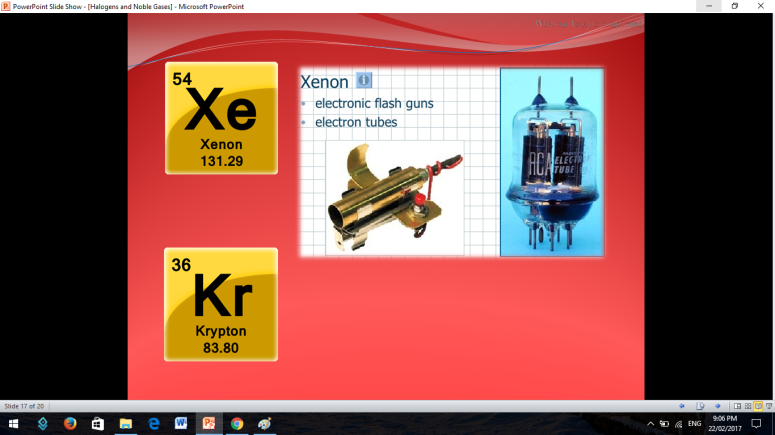
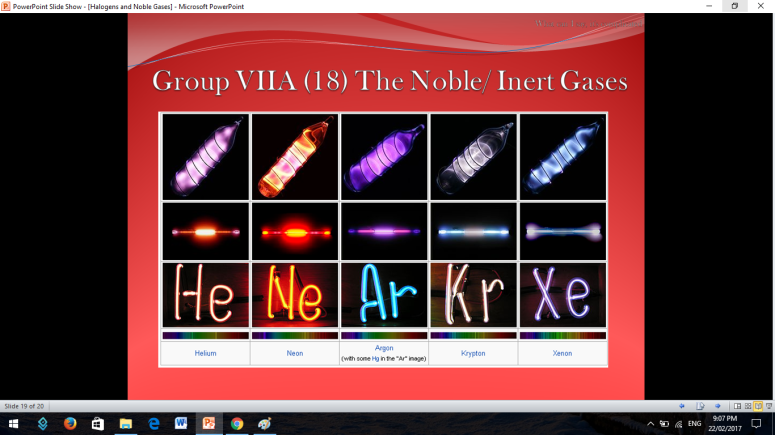
Similar to Halogens, Noble or Inert Gases are also non-metals wherein their outermost electrons in their electron configuration is now 8 (ns2np6). They are extremely stable monoatomic gases in nature. They exhibit little or no reactivity at all; although compounds of Krypton and Xenon have been prepared. So the noble gases are Helium, Ne on, Argon, Krypton, Xenon, and Radon.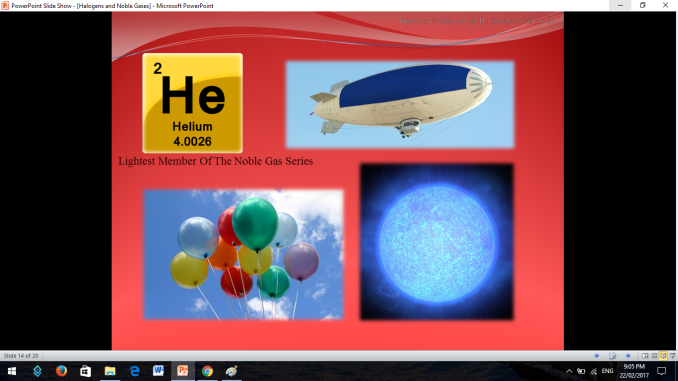


So there you have it: The Halogens and the Noble Gases
Greetings Fellow Netizens…
We are humbly asking for your support by means of liking, spreading or substantially commenting on our blog entitled “Getting To Know About Halogens And Noble Gases”
This topic would be a great help to those who want to know about CHEMISTRY.
 Thank You in Advance since your comments will be part of our grades… Thank you again and God Bless.
Thank You in Advance since your comments will be part of our grades… Thank you again and God Bless.
To support our fellow Stem-C as a whole you can visit these websites that would contain various topics, still representing and integrating hypertext or hypermedia.
https://stemcrwsg2.wordpress.com/
Entitled, “The K-Drama Fever”
This blog is for those who are K-Drama Fanatics.
https://stemcrwsblog02.wordpress.com/
Entitled, “The Twelve Olympian Gods”
This blog on the other hand is for those who has a thing about GREEK MYTHOLOGY.
The Author:
Reynald Bautista
STEM-C Group 4 Members and Supporters
Mr. Bade; Mr. Cervantes; Mr. Chavez; Mr. Maunes; Mr. Samoy;
Ms. Etrata ; Ms. Nerpiol; Ms. Gornez
Output in READING AND WRITING SKILLS








